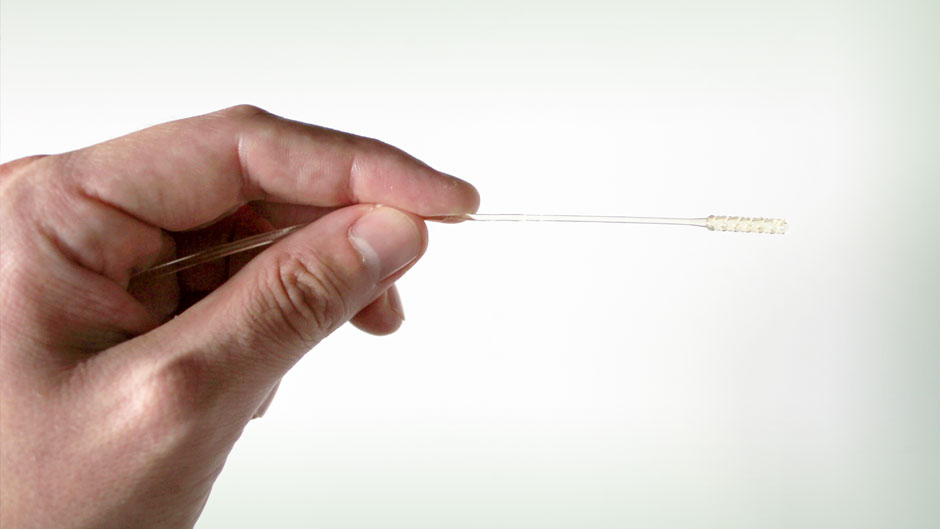
Long before a ventilator or therapies such as remdesivir and steroids are deployed, a 6-inch-long, skinny, flexible plastic stick is often the first line of defense in the fight against COVID-19.
The nasopharyngeal swab, which is inserted deep into the nasal cavity, remains the primary diagnostic tool used to test for the virus. But as screenings ramp up amid a massive spike in coronavirus cases in dozens of states, frontline health care workers are concerned that supplies of the highly specialized swabs will again run dangerously low.
Now, a University of Miami initiative that uses additive manufacturing is helping to allay those fears.
Biomedical engineers and architects at the University are in the homestretch of an ambitious project to 3D-print 1,000 nasopharyngeal swabs a day, doing their part to help maintain the stockpile of these crucial tools that are being used by the tens of thousands at hospitals, community health clinics, and drive-through test sites each week.
“As the virus started to gain a foothold locally and around the country, we knew that nasopharyngeal swabs would be in high demand and that the supply would start to run low,” said Ramon Montero, an assistant professor of professional practice in the biomedical engineering department at the College of Engineering. “We also knew we had the 3D-printing technology to produce them. It was just a matter of working with our doctors to make sure we designed what they needed.”

Montero, who is part of the University’s COVID-19 Preparedness Task Force, an endeavor aimed at 3D-printing and fabricating devices and personal protective equipment (PPE) for medical personnel on the front lines of the war against the coronavirus, started working on a nasopharyngeal swab design in late March.
University of Miami Health System pathologists Merce Jorda and Oleksandr Kryvenko were pleased with the initial design, but there was just one problem: The swabs were too flexible. While they had to be somewhat bendy, they still needed to be rigid enough to reach the back of the nasal passageway, where it meets the throat, to collect a sample.
“We had watched a video of how the swab testing is done and made the incorrect assumption that they had to bend a lot. But that just wasn’t the case at all,” Montero explained.
So his team went back to the drawing board, designing a swab that was stiffer and included small holes at the tip to facilitate specimen collection. “We finally came up with something that was just right,” Montero said.
They are using an autoclaving process—superheating the swabs at 121 Celsius for 30 minutes—to sterilize the instruments.
Plastics generally cannot withstand such an intense sterilization process, so Montero found a substance that could —a type of guidewire polymer used in surgical procedures like catheter placements.
Now the swabs are at the trial phase. They have already been tested on manikin and cadaver heads. Testing on human subjects comes next. But for that, Montero and his team will need FDA approval. They have applied for emergency use authorization from the FDA for the swabs, which accelerates access to critical medical products that may help during crises like the current pandemic.
“Our goal is to produce 1,000 swabs a day using as many as three 3D printers,” said Maxwell Jarosz, architect and manager of the fabrication lab and model shop at the School of Architecture, where many of the prototype swabs have been printed.
Others have been produced at the College of Engineering–Johnson & Johnson 3D Printing Center of Excellence Collaborative Laboratory.
If FDA approval is secured, swabs used for actual patient testing will be 3D-printed at UHealth Tower. “Our plan is to help the hospital install its own printer for production once the design is ready,” Jarosz said.
As the swabs begin to be mass-produced, Montero and Jarosz will work with second-year Miller School of Medicine student Alison Ohringer, who could play a role in expanding distribution to other frontline health care workers.
Ohringer founded and serves as the president of Miami Med COVID Help, a nonprofit organization with about 150 Miller School student volunteers that coordinates donations of PPE and other supplies to area hospitals.
She, along with Jarosz, sits on the COVID-19 Preparedness Task Force. Face shields, surgical helmets, filter caps for N95 masks, and intubation boxes that protect health care workers from aerosolized particles produced during endotracheal intubation are among the items the University-wide task force has helped produce.